Definition:
Methanol (CH3OH) is the simplest alcohol molecule. It has many applications and is mostly used in chemicals and industrial areas. But it also has potential as a fuel, through combustion reaction:
(Image Credit: ^3)
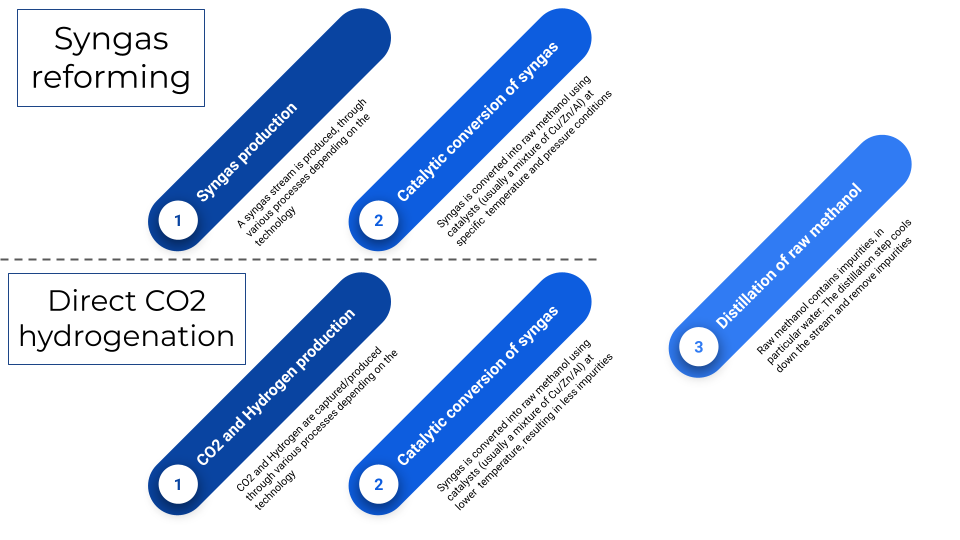
Methanol production can be separated into 2 kinds of processes ^2 : * through syngas reforming: in(syngas), out(methanol, water) [NOT IMPLEMENTED YET] * through direct CO2 hydrogenation: in(carbon dioxide, hydrogen), out(methanol, water)
Although, usually plants are constructed to take a source fuel (biomass, natural gas,...) to produce the syngas on site.
Significant datas for liquid fuel ^1:
- Chemical Formula : CH3OH
- Molar mass : 32.04 g/mol
- Density: 792 kg/m^3
- CO2 after use : 1.37 kg/kg
- CH4 after use : 0.0 kg/kg
- N2O after use : 0.0 kg/kg
- High Calorific value : 6.39 kWh/kg
The advantages of Methanol as a fuel^3:
- It could be used in the auto industry without having to replace the current thermic engine automobile fleet
- It is liquid at room temperature and thus easier to store
The disadvantages of Methanol as a fuel:
- It is less dense than other liquid fuel (diesel for example) and would thus require more fill-ups
- The lesser lubrification compared to other oils may increase the wear and tear costs
^3 Schröder, J., Müller-Langer, F., Aakko-Saksa, P., Winther, K., Baumgarten, W. and Lindgren, M., 2020. Methanol as motor fuel: Summary Report.
^4 Nyári, J., 2018. Techno-economic feasibility study of a methanol plant using carbon dioxide and hydrogen.