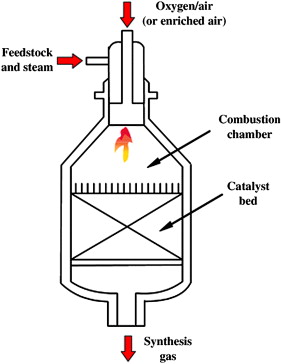
Autothermal reforming
Autothermal reforming uses CO_2 and oxygen in a reaction with methane to form syngas. The reaction takes place in a single chamber where the methane is partially oxidized. The reaction is exothermic due to the oxidation.
The syngas produced ratio of H2:CO is 1:1.
Economic and technical datas was taken from Ayodele et Al. ^1 & Cormos et Al. ^2.
^1: Freida Ozavize Ayodele , Siti Indati Mustapa , Bamidele Victor Ayodele and Norsyahida Mohammad (2020) An Overview of Economic Analysis and Environmental Impacts of Natural Gas Conversion Technologies
^2: Ana-Maria Cormos et Al. (2018). Economic Assessments of Hydrogen Production Processes Based on Natural Gas Reforming with Carbon Capture